
Black layer from a creeping bentgrass putting green in northern Michigan. Photos by William Berndt
After 30 years, black layer has again reared its ugly head. Articles on the subject have cropped up recently, as have discussions about it on social media. Much of the information about black layer is anecdotal, based on observations. This article aims to clarify what black layer is and how it can be managed, based on university research.
What is black layer?
Physically, black layer is an accumulation of metal sulfides in the pore space of soil. Metal sulfides form when hydrogen sulfide (H2S) reacts with metals such as iron (Fe2+), producing iron sulfide (FeS):
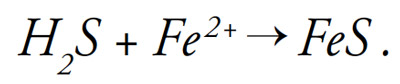
Sulfur-reducing bacteria (SRB) generate hydrogen sulfide during respiration:
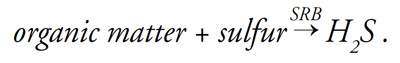
This process requires the presence of sulfur in the same way that animals require oxygen. Hydrogen sulfide is released only when soil redox potential is low (that is, when soil is anaerobic).
To verify that black layer is composed of metal sulfide, black layer samples from 26 different putting greens in Michigan and Ohio were spot-tested with a solution of sodium azide (NaN3-) and iodine (I2) (1). This solution is nonreactive except in the presence of metal sulfide (MeS), which acts as a catalyst to produce sodium iodide (NaI) while releasing nitrogen gas (N2):
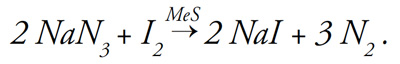
To test for the presence of metal sulfide, black layer soil was added to the solution. If nitrogen gas (N2) was released (as evidenced by fine bubbling) when the black layer was added, metal sulfide was present. Every one of the 26 black layers examined tested positive for metal sulfide (1). It is important to know that the presence of metal sulfides (that is, black layer) in a putting green is proof that soil redox potential is low and that hydrogen sulfide is being generated.
What causes black layer turf decline?
Hydrogen sulfide is probably what causes the turfgrass decline associated with black layer, although other compounds associated with low redox potential may also be toxic to turf. (Low redox potential may also initiate ethylene production, produce carboxylic acids, and make manganese available, all of which can be toxic.) Hydrogen sulfide is well known as a colorless gas that has the smell of rotten eggs. It is also a poison that disrupts cellular respiration in both plants and animals, and it has been associated with akiochi disease in rice (8) and injury in citrus tree roots (7), which suffered toxicity at a hydrogen sulfide level of 2.8 ppm.
The generation of hydrogen sulfide is biological. To demonstrate this, researchers used radioactive sulfate as a tracer directly in black layer (4). When intact cores of a black layer from a Penncross creeping bentgrass (Agrostis stolonifera L.) green were injected with radioactive sulfate, it converted to radioactive sulfide at a rate of 3 to 14 nanomoles of sulfur per cubic centimeter of soil per day. About 32% of the radioactive sulfate was converted to radioactive sulfide, and 80% to 95% of the radioactive sulfide was metal sulfide (Figure 1, below). When the radioactive sulfate was injected into cores along with sodium azide, which is a soil sterilant, almost no radioactive sulfide was produced — the biological production of sulfide was halted by the azide. And when the radioactive tracer was injected with sodium molybdate, which is a specific inhibitor of biological sulfur reduction, again almost no sulfide formed. This research showed that hydrogen sulfide actively formed in an existing black layer, and that it was a biological process.
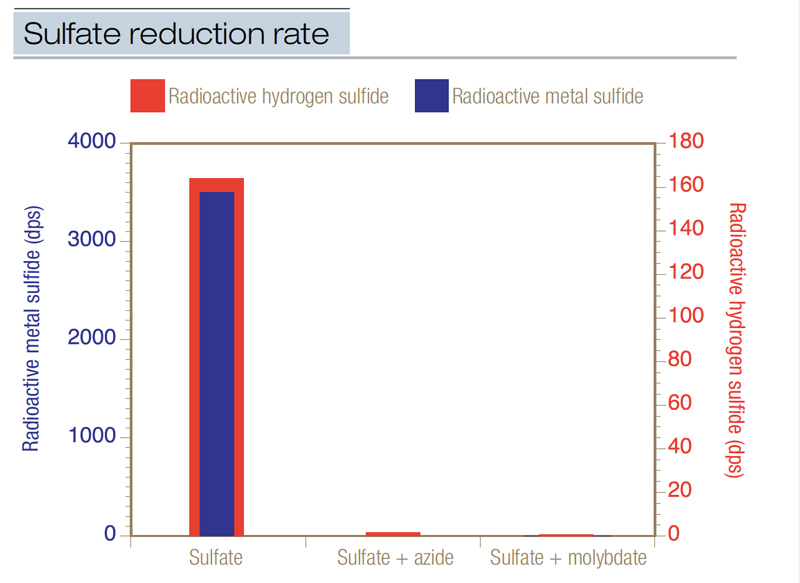
Figure 1. Radioactive sulfate (35SO42-) was used to calculate the rate of sulfate reduction (expressed in dps, disintegrations per second) in intact cores taken from an existing black layer in a Penncross creeping bentgrass green. When radioactive sulfate was injected into intact cores, it was reduced at a mean rate of 7.1 nanomoles sulfur/cubic centimeter of soil/day. Both hydrogen sulfide and metal sulfide were produced at high levels. When radioactive sulfate was injected concurrently with sodium azide, the sulfate reduction rate declined to 0.03 nanomole sulfur/cubic centimeter of soil/day. When radioactive sulfate was injected concurrently with sodium molybdate, the sulfate reduction rate was 0.01; very little sulfide was produced. This data was the first proof that biological reduction of sulfate produces sulfide in a black layer from a creeping bentgrass green.
The research also demonstrated that hydrogen sulfide is toxic to turfgrass. Established cores of Penncross creeping bentgrass were exposed to hydrogen sulfide at a level of 1,000 ppm for a period of seven days (1). After only 12 hours of exposure, the aerial tissue was visibly stunted. After seven days, the bentgrass was dead, with turf turning from deep green to straw brown. Root tissue was also visibly stunted and appeared necrotic compared with the control. Thatch and exposed roots took on a blackened appearance characteristic of black layer. Based on these results, and on the fact that the presence of metal sulfide in black layer proves that hydrogen sulfide is being generated, it is reasonable to conclude that the turf loss is at least partially a result of hydrogen sulfide toxicity.

The effect of hydrogen sulfide on creeping bentgrass turf after seven days. Left: Control turf with no exposure to hydrogen sulfide. Right: Turf exposed to 1,000 ppm hydrogen sulfide for seven days.
What makes soils anaerobic?
Waterlogging or excessive soil microbial activity can induce anaerobic conditions in soil. Waterlogging from excessive rainfall or irrigation physically restricts oxygen diffusion into soil (Table 1). Development of physical layers in the root zone from topdressing can also hinder oxygen diffusion. Other factors that influence oxygen diffusion are soil pore size distribution, organic matter content and compaction. Too many fines in greens mix and too much organic matter can increase soil’s water-holding capacity. Compaction, which is the consolidation of pore spaces, can directly restrict both drainage and gas exchange. If oxygen diffusion becomes inhibited for any reason, then microbial respiration can deplete available soil oxygen within a few hours. Irrigating judiciously, ensuring that drainage is adequate, alleviating compaction via cultivation, and paying close attention to greens mix properties are vital considerations for preventing low redox potential.

Measurement of oxygen diffusion with platinum electrodes in a northern Michigan putting green afflicted with black layer. See Table 1 for data relevant to this photo.
Low redox potential can also be induced through certain cultural practices. Applying soil amendments such as elemental sulfur or fertilizers such as natural organic sources of nitrogen can deplete soil oxygen, especially if oxygen diffusion is restricted. Consider that natural organic fertilizers must react chemically with oxygen to produce nitrate:
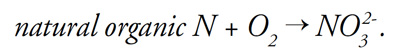
This process, known as “nitrification,” binds oxygen (O2) to nitrogen, creating nitrate and making oxygen as O2 less available.
Applying elemental sulfur or products containing sulfur also contributes to low redox potential by binding available oxygen:
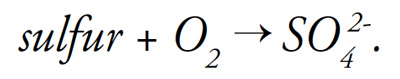

Topdressing layers in the soil profile of a putting green. Layering is a physical problem that can restrict drainage and gas exchange.
Adding elemental sulfur to soil lowers redox potential and drives production of hydrogen sulfide (2,6). Treating putting green sand with elemental sulfur at a rate of 1.5 pounds/1,000 square feet (7.3 grams/square meter) and then waterlogging the sand for 21 days significantly reduced redox potential and resulted in the generation of 4 ppm to 5 ppm free hydrogen sulfide (Figure 2, below). Increasing the rate to 3 pounds/1,000 square feet (14.6 grams/square meter) had little additional effect on either redox potential or hydrogen sulfide generation, except when supplemental organic matter was present. If elemental sulfur and organic matter were present at relatively high levels, then 20 ppm to 30 ppm free hydrogen sulfide was generated, and 65 ppm to 100 ppm metal sulfide was formed. As relatively high levels of organic matter are typically present in putting greens, applying elemental sulfur would be contraindicated, as it can lower redox potential and, in turn, drive the release of hydrogen sulfide, which may result in black layer formation and a decline in turf quality.
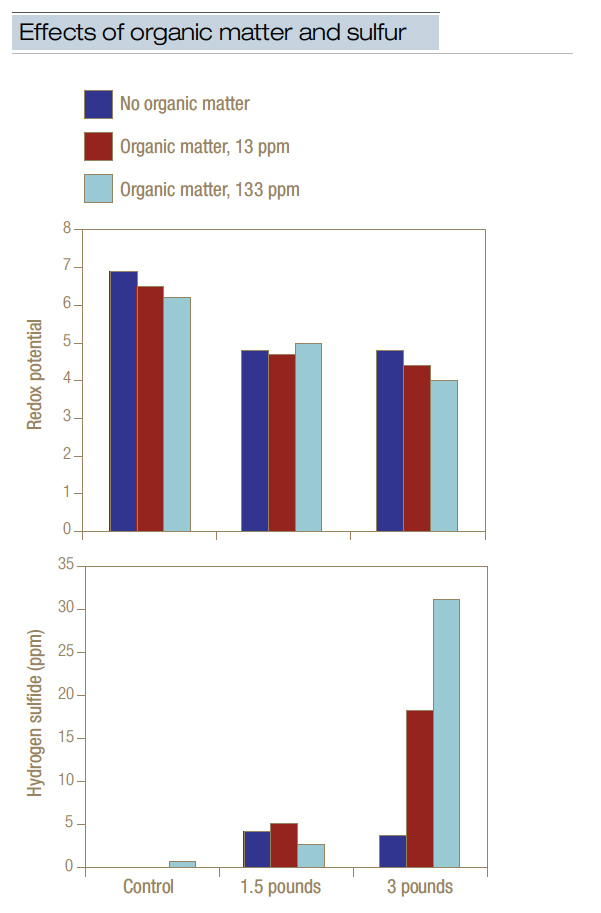
Figure 2. Treating flooded putting green sand with elemental sulfur and/or organic matter depressed redox potential and stimulated accumulation of free hydrogen sulfide. Sulfur applied at 1.5 pounds/1,000 square feet (7.3 grams/square meter) significantly lowered redox potential and generated hydrogen sulfide. This data implied that applying sulfur to sand-based putting greens may induce low redox potential (that is, anaerobic conditions) and the subsequent release of hydrogen sulfide. The data also demonstrated the importance of organic matter to the process.
How can black layer be controlled?
Managing black layer means keeping redox potential elevated and withholding the application of sulfur or sulfur-containing products. Fertilizing with nitrates would be a best management practice for keeping soils aerobic. Nitrates are powerful oxidizing agents that are known to elevate redox potential in soils. Research was conducted to determine whether nitrate could be used to keep redox potential elevated in black layer soil and in turn prevent the release of hydrogen sulfide (3). When saturated turfgrass soil was treated with elemental sulfur at 1 pound/1,000 square feet (4.8 grams/square meter), redox potential was depressed and total sulfide accumulated to near 60 ppm (3). When nitrate was applied at 1 pound/1,000 square feet concurrently with sulfur, redox potential was elevated and sulfide accrual was reduced below the level observed for the control (3).
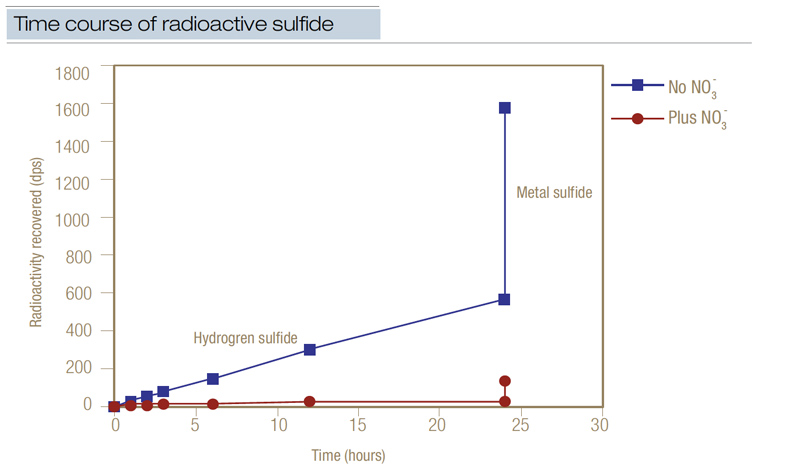
Figure 3. Radioactive sulfate (35SO4-2) was used to chart the time course of the appearance of radioactive sulfide over 24 hours in disturbed soil from a Penncross creeping bentgrass green. (Radioactivity recovered was measured in dps, disintegrations per second.) Where nitrate was added, the mean residence time of the sulfur pool increased from 6.6 days to 75.9 days, while the residence half-life of the radioactive label increased from 3.5 days to 40.2 days. This data demonstrated that when nitrate was applied at 1 pound/1,000 square feet (4.8 grams/square meter), it effectively reduced the accumulation of sulfide.
In supporting research, radioactive sulfate was used to chart the time course of the appearance of hydrogen sulfide in disturbed black layer soil from a Penncross creeping bentgrass green (5). Without the addition of nitrate, radioactive sulfide was generated at a mean rate of 53 nanomoles sulfur/cubic centimeter/day (Figure 3, above). When nitrate was added at 1 pound/1,000 square feet concurrently with the radioactive sulfur, sulfide was generated at only 4 nanomoles sulfur/cubic centimeter/day. About 6.5% of the added radioactive sulfur turned into radioactive hydrogen sulfide in only 24 hours, while 25% of the radioactive label became radioactive metal sulfide.
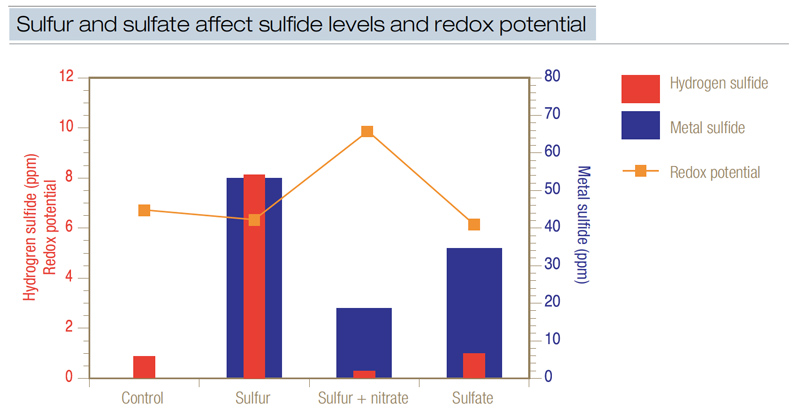
Figure 4. The influence of sulfur and sulfate on the production of hydrogen sulfide, metal sulfide and soil redox potential. When redox potential is low, relatively high levels of sulfides are produced when sufficient sulfur is present. Sulfur as elemental sulfur or sulfate was applied to flooded sand at 1 pound/1,000 square feet (4.8 grams/square meter). Nitrate as calcium nitrate [Ca(NO3)2] was applied to provide nitrogen at 1 pound/1,000 square feet. This data demonstrated that nitrate applied at 1 pound/1,000 square feet effectively reduced sulfide accumulation by increasing redox potential to a point high enough to avert sulfide formation.
Additional research (6) again demonstrated that adding 1 pound of elemental sulfur or the equivalent rate of sulfur from sulfate generated both free hydrogen sulfide and metal sulfide (Figure 4). Adding sulfur or sulfate generated 3 to 8 ppm hydrogen sulfide. When sulfur was added concurrently with nitrate, hydrogen sulfide production was reduced because redox potential was elevated.
Conclusions
Black layer is an accumulation of metal sulfides in pore spaces of soil. Its presence is an indicator that redox potential is low and that hydrogen sulfide is being released. Hydrogen sulfide is toxic to turfgrass plants, and it is a likely cause of the turf decline associated with black layer. Low redox potential occurs when oxygen diffusion is limited for any reason, including from waterlogging or the presence of soil layers.
Cultural practices such as applying elemental sulfur to lower pH or applying sulfur-containing products can induce low redox potential by binding available oxygen. Adding supplemental organic matter (that is, natural organic nitrogen) can also induce low redox potential via nitrification. If redox potential becomes low enough, the presence of sulfur will drive production of hydrogen sulfide through the respiratory activities of sulfur-reducing bacteria. The principle to remember here is that black layer forms only when redox potential is low. Employing cultural practices that keep redox potential elevated, such as fertilizing with nitrate, withholding sulfur and managing water, are considered best management practices for dealing with black layer.
The research says ...
- Black layer, an accumulation of metal sulfides in pore spaces of soil, indicates that redox potential is low and that hydrogen sulfide is being released.
- Waterlogging or the presence of soil layers can induce low redox potential.
- Applying elemental sulfur or sulfur-containing products and adding supplemental organic matter (natural organic nitrogen) can induce low redox potential.
- Cultural practices such as fertilizing with nitrate, withholding sulfur and managing water keep redox potential elevated and thus help prevent black layer.
Literature cited
- Berndt, W.L. 1990. Investigations into turfgrass black layer. Ph.D. dissertation, Michigan State University, East Lansing, Mich.
- Berndt, W.L., and J.M. Vargas Jr. 1992. Elemental sulfur lowers redox potential and produces sulfide in putting green sand. HortScience 27:1188-1190.
- Berndt, W.L., and J.M. Vargas Jr. 1996. Preventing black layer with nitrate. Journal of Turfgrass Management 1:11-21.
- Berndt W.L., and J.M. Vargas Jr. 2006. Dissimilatory reduction of sulfate in black layer. HortScience 41:815-817.
- Berndt, W.L., and J.M. Vargas Jr. 2007. A review of the nature and control of black layer. Dynamic Soil, Dynamic Plant 1(1):17-23 (www.globalsciencebooks.info/Online/GSBOnline/OnlineDSDP_1_1.html). Accessed May 4, 2016.
- Berndt, W.L., and J.M. Vargas Jr. 2008. Elemental sulfur reduces to sulfide in black layer soil. HortScience 43(5):1615-1618.
- Ford, H.W. 1973. Levels of hydrogen sulfide toxic to citrus root. Journal of the American Society of Horticultural Science 98(1):66-68.
- Vamos, R. 1958. H2S, the cause of the bruzone (Akiochi) disease of rice. Soil Science and Plant Nutrition doi:10.1080/00380768.1958.10431937. Accessed May 4, 2016.
William L. Berndt is a former faculty member at Edison State College and Florida Gulf Coast University, and is currently the president of Environmental Turf Inc. in Avon Park, Fla.